The toxicity of an element or a compound depends on the species (or forms) it exists as. Understanding and identifying different chemical species is fundamental to addressing their impact on health and environment.
When we hear the word ‘toxic’, certain chemical elements might immediately spring to mind. Lead, mercury and arsenic are classic examples of elements that you would not want to touch, ingest or inhale. They are toxic, meaning they can poison our bodies. However, the toxicity of an element depends on the ‘species’ it exists as (i.e. Chemical Speciation). A chemical species is a particular elemental form with a characteristic isotopic form, electronic and oxidation state (charge) and/or relationship with other elements. For example, arsenic can exist as arsenite (trivalent) or arsenate (pentavalent) in a variety of inorganic or organic species (see Fig.1 below). These inorganic arsenic species found in contaminated water and soils are highly toxic compared to organic arsenic compounds found in seafood.
Speciation is the distribution of an element across different chemical species. If we understand the behaviour and toxicity of the various species of a chemical element and the environmental conditions in which they exist, we can better determine their impacts on health and the environment.
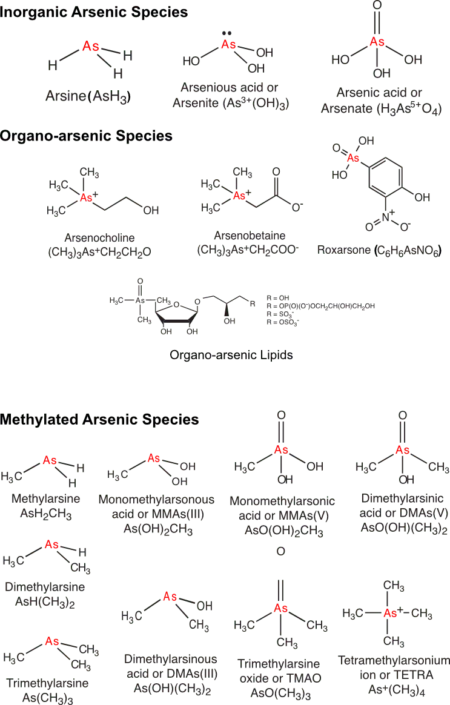
Fig. 1: Compilation of selected arsenic inorganic and organic species found in water, food and soils.
1. What Causes Chemical Speciation?
There are several different physical and biogeochemical processes which can cause a chemical element to speciate, including any, or a combination, of the following:
- Loss or gain of neutrons caused by radioactive decay or other physical, chemical or biological processes which change the isotopic composition of the element
- Chemical reactions which involve oxidation or reduction or changes to the alkalinity, pH, temperature and pressure of the environmental conditions
- Biological processes such as in soil and living organisms which use oxidation, reduction, hydrolysis or conjugation to degrade chemicals and access nutrients
2. Why Are Some Chemical Species More Toxic Than Others?
The chemical characteristics of a certain species determine how dangerous it is. For example, metals and metalloids generally have multiple possible species, allowing them to bind to biomolecules such as proteins, carbohydrates, lipids and nucleic acids. Metals such as iron, copper and zinc are involved in essential biochemical reactions, which means they are crucial to our health and the proper functioning of our bodies. However, most can also be toxic when they exist as certain species and at high levels.
The oxidation state, inorganic bonding (bonding without carbon atoms involved) and organometallic bonding (bonding between metals and carbon-bearing groups) of a metal element species are the most significant characteristics affecting the element’s toxicity and accessibility to biomolecules like proteins (bioaccessibility). These factors determine whether the metal poses a risk to our health.
For example, iron is an essential element for almost all living organisms. However, iron species such as divalent iron, Fe(II) are known to cause neurotoxicity in humans and root toxicity in plants.
Chromium is a common toxic substance. Trace trivalent chromium, Cr(III), is non-toxic and essential for proper protein, fat and carbohydrate metabolism. However, hexavalent chromium, Cr(VI), is a well-recognised human carcinogen and potent neurotoxic, generating free radicals that cause DNA damage. Chromium preferentially exists as Cr(III) in highly reducing conditions and Cr(VI) in highly oxidising conditions.
Chemicals present as solid compounds such as nickel sulphides and oxides have limited bioaccessibility because they are insoluble. However, if nickel bonds with organic molecules, it can form soluble compounds. These are more likely to inhibit the function of biomolecules and cells, causing harm to living organisms and essential organs in our body.
3. Why is Knowledge of Chemical Speciation Important?
Understanding how chemicals speciate is fundamental to understanding toxicity, bioaccessibility, bioavailability and the fate of toxic elements in the environment. Developing therapeutic approaches for metal poisoning, knowing how toxic elements damage living organs and effect plant growth and tracing how they enter the food chain are all exercises in chemical speciation.
For example, in the case of the recent oil spill off the coast of Mauritius, understanding how mercury from the oil will speciate in the water, soil and biological systems of the island allows us to evaluate its potential ecological impact. This means we can plan an appropriate response.
Chemical speciation analysis, rather than traditional total content measurements, is therefore fundamental to understanding the mobility and toxicity of elements and to the development of proper environmental and food regulations. However, current analytical tools available for determining chemicals species in samples are complex and expensive and rely on highly skilled operators. Nanolyse Technologies Ltd aims to solve challenging problems related to the separation, determination and monitoring of toxic inorganic and organometallic chemical species.
Please cite this article as: “Matt Randall, Bàrbara Levering and Juliet Martin (2020), Chemical speciation: what it is and why it matters. Nanolyse Technologies, Oxford, UK.”
Further Reading
- Templeton, Douglas M., et al. (2000), ‘Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000)’, Pure and Applied Chemistry, 72(8) (8), https://doi.org/10.1351/pac200072081453
- de Jesus J.R., da Costa L.F., Lehmann E.L., Galazzi R.M., Madrid K.C., Arruda M.A.Z. (2018) Chemical Speciation and Metallomics. In: Arruda M. (eds) Metallomics. Advances in Experimental Medicine and Biology, vol 1055. Springer, Cham. https://doi.org/10.1007/978-3-319-90143-5_8
- World Health Organization. (2006). Elemental speciation in human health risk assessment / authors, P. Apostoli … [et al.]. World Health Organization. https://apps.who.int/iris/handle/10665/43442